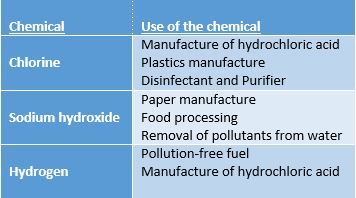
Brine in Chemistry:Electrolysis:The process of electrolysis uses an electric current to create a chemical change. Brine is commonly used in electrolysis as chlorine gas is manufactured from the chemical reaction. Two other chemicals are also obtained during the process, sodium hydroxide (NaOH) and hydrogen (H2)
Chlorine, sodium hydroxide, and hydrogen have several uses: Drying Organic Solutions:Synthesizing and isolating organic compounds results in the solution sometimes being contaminated with traces of water. This water must be removed from the required compound so it can be properly characterised.
Washing the organic layer with brine can remove the bulk of the water. The salt water pulls the water from the organic layer to the water layer. This is due to salt being more hygroscopic than the organic compound. |
Further Info: |